By Ron Goldy, Michigan State University Extension
Improper pH and higher than adequate nutrient levels are reasons for regular soil testing.
There are currently 20 nutrients known to be essential for plant growth (Table 1). Carbon, hydrogen and oxygen are obtained from air or water while others are obtained from the growing media, whether that is soil in the field, a hydroponic system or something in between. Nutrients obtained from air and water are largely beyond producer control, although in greenhouse situations CO2 levels can be enhanced, but those obtained from the growing media can, in most situations, be adjusted if needed.
Table 1. List of the 17 nutrients essential for plant growth, their Periodic Table symbol and where the nutrient is generally obtained. |
|---|
Primary | Symbol | Source |
|---|
Nitrogen | N | Soil |
Phosphorous | P | Soil |
Potassium | K | Soil |
Secondary | Symbol | Source |
|---|
Calcium | Ca | Soil |
Magnesium | Mg | Soil |
Sulfur | S | Soil |
Micronutrients | Symbol | Source |
|---|
Boron | B | Soil |
Chlorine | Cl | Soil |
Cobalt | Co | Soil |
Copper | Cu | Soil |
Iron | Fe | Soil |
Manganese | Mn | Soil |
Molybdenum | Mo | Soil |
Silicon | Si | Soil |
Sodium | Na | Soil |
Vanadium | V | Soil |
Zinc | Zn | Soil |
Others | Symbol | Source |
|---|
Carbon | C | Air |
Hydrogen | H | Water |
Oxygen | O | Air/Water |
Most producers are aware soil pH influences nutrient uptake and have seen Figure 1. This figure shows that if soil pH is not within the proper range – 6.2 to 7.2 for most crops – nutrient uptake is inhibited. That doesn’t mean the nutrient is not in the soil, it just means the soil chemical environment is not suitable for uptake of that nutrient. This usually takes place in highly alkaline (greater than 7.5) or highly acidic (less than 5.5) situations. If the pH is outside the desired range, recommendations will be made to either add sulfur to lower pH or lime to raise it. High organic soils, however, are well buffered and resistant to pH changes so plants grown in these soils will generally need foliar applications of limiting elements. Outside the desired pH range it is also possible for some non-essential nutrients to become more available, which can lead to nutrient toxicities. Aluminum (Al) is best known for this at lower pH.
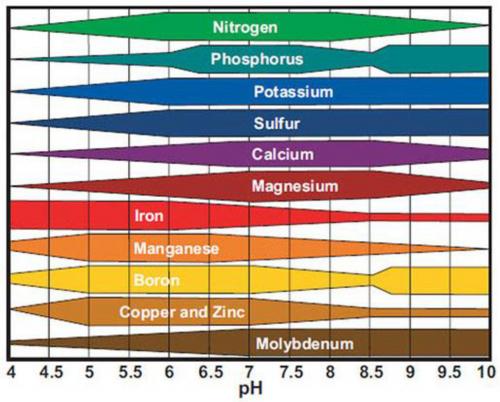
Figure 1. Nutrient availability in relation to pH. The thicker the bar the more available the nutrient.
A lesser known but equally important interaction is the one shown by the Mulder’s Chart (Figure 2). The Mulder’s chart represents the interaction between 11 of the essential plant elements. Some interactions are positive (synergistic) and others are negative (antagonistic). A synergistic relationship is one where the elements involved help each other by aiding uptake or utilization. In contrast, an antagonistic relationship means the elements hinder each other in uptake or utilization. For example, adequate potassium aids in use of iron and manganese, but if it is too high it will hinder (antagonizes) utilization of magnesium, boron, nitrogen, phosphorous and calcium. An antagonized element may be present in adequate levels, but there is so much potassium present the plant doesn’t have access to it. Elements that act as antagonists can do so in a couple ways. If calcium is in excess it can simply out-compete other elements such as potassium and magnesium for uptake sites on the roots, or it can change soil chemistry by elevating pH to the point iron and boron become unavailable.
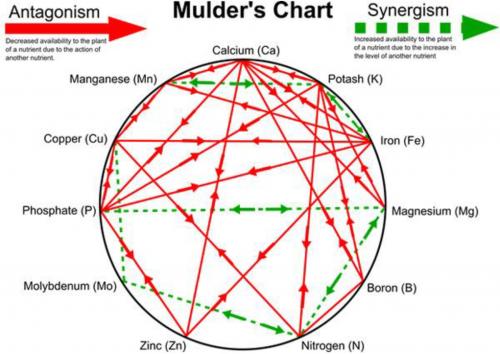
Figure 2. Mulder’s chart of antagonistic (solid lines) and synergistic (dashed lines) elements.
Improper pH and higher than adequate nutrient levels provide more reasons for the need for regular soil testing. To apply nutrients without first establishing a base nutrient level is one of the biggest mistakes growers can make. I have known growers who have applied potassium without soil tests only to have their plants show symptoms of magnesium deficiency. Farming is risky enough, so don’t ignore the simple and inexpensive step of performing a soil test.