Potassium (K) and base saturation
Potassium tends to be the major nutrient that is discussed when it comes to cation saturation ratios. For potassium, the data shows that maintaining an optimal soil test can achieve high yield potential without focusing on the amount of the CEC occupied with potassium. There are a couple issues with using cation saturation ratios:
#1 Achieving an “optimal” K base saturation ratio can be difficult on soils high in clay
Clay content tends to be one of the largest factors impacting the K base saturation ratio. I have heard claims that a response to K is highly likely when the K base saturation ratio is less than 4%, which to me is extremely high. So, let’s investigate this a bit and what it takes to get a K base saturation ratio of 4%.
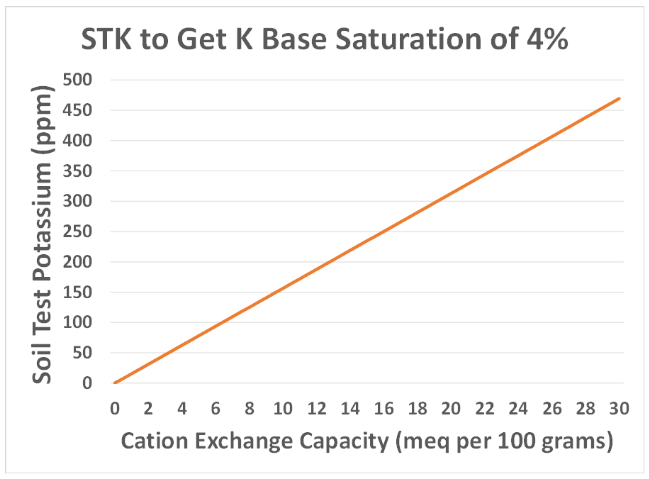
The figure above shows the soil test needed to get to a base saturation of 4% for various CEC values, which are measured in milliequivalents (meq) per one hundred grams of soil. A milliequivalent is a measure of charge and is based on the charge of an ion, which in the case of potassium is plus one. If we look at a Hubbard loamy sand from near Becker, Minnesota with a CEC of around 5, it would take a soil test K concentration of 78 ppm to get to 4% K base saturation. Compare that to a Normania loam soil from Lamberton, Minnesota with a CEC of 25, which needs a soil test K concentration of 391 ppm to get to 4% K base saturation. Therefore, it is a lot easier to get to 4% on the sandier soil versus a loam soil, and I think the major reason many people are recommending higher K base saturations is that they may be looking at yield achieved in, say, a sandy irrigated soil compared to a higher clay non-irrigated soil and claiming that the K base saturation ratio being different is the reason for the yield difference. This would be possible if the only factor influencing the maximum yield is K, but that is not likely the case.
Source : umn.edu