According to the Centers for Disease Control and Prevention (CDC), the “slow moving” pandemic of antibiotic resistance is best addressed using a “One Health” approach. That kind of an approach, for example, would be one that holistically tracked antibiotic use, along with levels of resistance, in human populations, in animals, and in the environment. Since 2009, the FDA has reported annual sales of livestock antibiotics but no information on veterinary prescriptions or orders; human sales data also are not reported. Meanwhile, the CDC now issues annual reports on antibiotic prescriptions in human medicine, but these releases do not provide national data on medical antibiotic sales.
Our New Analysis. For the third time since 2017, NRDC and Center for Disease Dynamics, Economics & Policy (CDDEP) have teamed up to fill in critical data gaps around antibiotic use in the United States. Table 1 and the accompanying figure include both the FDA’s annual data on livestock sales of medically important antibiotics for 2009 to 2019, along with estimated use of the same drugs in human medicine. To estimate human use, CDDEP used data obtained from IQVIA, a private company that directly collects outpatient antibiotic prescribing information across the U.S. Total medical use of antibiotics was approximated by combining outpatient data with estimates of hospital prescriptions.
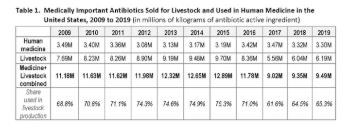
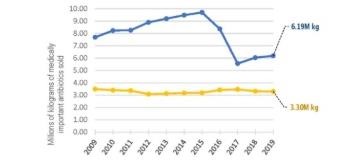
Doing a side-by-side comparison of the same medically important antibiotics sold for livestock use and used in human medicine allows for better appreciation of key differences and patterns. Use of antibiotics in human medicine has remained consistent since 2009. It even declined slightly since 2017, despite the year-on-year increase in the U.S. population. By contrast, sales of these same drugs for use in livestock increased 11.3% from 2017 to 2019, from 5.6M kg to 6.2M kg. Livestock sales are now nearly double the sales for human medicine (6.19M kg vs. 3.30M kg).
From 2009, livestock antibiotic sales increased to a peak in 2015, before falling in 2016 and 2017. This was the period when FDA first urged the livestock sector to stop marketing and selling medically important antibiotics in animal feed for the purpose of growth promotion. Growth promotion use of feed antibiotics was made illegal in January 2017. All remaining feed uses were also put under veterinary oversight, meaning that a veterinarian has to write a prescription or veterinary “feed directive” before a feed mill will mix medically important antibiotics into animal feed and distribute it to producers. Under these feed directives, however, many livestock antibiotics continue to be fed to herds of animals on a routine basis for the so-called disease prevention, even when there are no sick or diseased animals.
The net increase in livestock sales since 2017 is entirely due to more antibiotics being sold for use in pig and cattle production. Over those two years, pig sales rose by about 560,000 kg, or nearly 28%, as illustrated in Table 2. Sales for cattle also rose, by a more modest 8%. Sales of medically important antibiotics for pigs and cattle combined are 55% higher than sales of those medicines for human patients.
Click here to see more...