By Elsa Sanchez, Thomas Ford, and Robert Berghage
Water quality has a large influence on crop growth and development.
We recommend that you evaluate your water supply prior to purchasing a farm or growing high value horticultural crops. In many areas of Pennsylvania, we have observed problems with sodium, chlorine, and/or boron levels, high alkalinity, and high electrical conductivity. For some of these issues there is no easy, low cost fix. Knowing about water quality issues before purchasing a farm or growing horticultural crops can be used for making purchasing and production decisions.
For example, high chloride and sodium levels, and sodium adsorbtion ratios often necessitate locating another water source. If this is not practical, diluting the existing water source with collected rainwater can reduce its sodium and chloride levels. Farmers in this situation have also explored reverse osmosis systems, but their cost of about $12,000 for 5,000 gallons of irrigation water per day are generally not practical for most high tunnel and commercial vegetable growers.
As with soils, pH is an important measure of irrigation water quality. pH is a measure of the relative acidity or hydrogen ion concentration in the water. The optimal range is 5.0 to 7.0. When evaluating irrigation water quality pH it is also important to consider the alkalinity of the water.
Alkalinity is a capacity measure. It measures the capacity of the water to neutralize acid. This is due primarily to the combined amount of carbonate (CO3) and bicarbonate (HCO3), but hydroxide, ammonium, borate, silicate, and phosphate can also contribute. Alkalinity is expressed in milligrams per liter (mg/L) on reports from the Lab and is provided as total alkalinity as CaCO3 (calcium carbonate), bicarbonate (HCO3) alkalinity and carbonate (CO3) alkalinity. When total alkalinity as CaCO3 is between 30 and 100 mg/L, we are not concerned.
When total alkalinity as CaCO3 is below 30 mg/L, the water has low buffering capacity. As a result, its pH will readily change depending on what is added to it. When total alkalinity as CaCO3 is above 100 mg/L, the pH of the water is high.
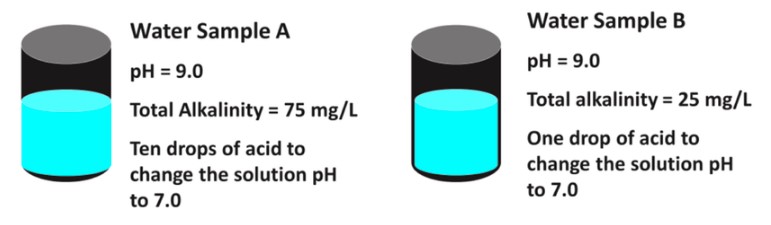
Acid can be injected with the irrigation water to decrease high pH water. The amount of acid needed depends on the alkalinity of the water. As a simple example, pretend that we have a container of water with a pH of 9.0 and a total alkalinity level of 75 mg/L. In this case the pH is high and the alkalinity level does not fall into the level of concern. It may take ten drops of acid to change the water pH to 7.0. Say another container of water also has a pH of 9.0, but a total alkalinity level of 25 mg/L. In this case the pH is high and the alkalinity level is low. It takes much less acid to decrease the pH, say 1 drop, because the water has a low buffering capacity. Even though both containers of water had identical pH (9.0), due to the alkalinity of the water, the amount of acid needed to change the pH is very different.
One of the farmers participating in our high tunnel project uses pond water for irrigating their crops. Analysis of an initial irrigation water sample showed the pH of the water to be 9.0. Generally, pond water has a pH between 6.0 and 9.0. However, the farmer also mentioned that their pond had a growth of filamentous algae. Because of this, we suspected that while the total alkalinity as CaCO3 did not fall with the level of concern, the water was poorly buffered.
The pH of poorly buffered pond water with algae will vary by time of day based on the rate of photosynthesis or respiration of the algae. We would expect it to be lower at daybreak and higher at mid-day, for example. A second irrigation water test taken at a different time of day, confirmed our suspicions and showed a pH of 8.0. In this case, liming can help buffer the pH of the water. The University of Florida has a fact sheet entitled
Use of Lime in Fish Ponds which includes information on liming ponds.
For the algae,
adding barley straw is an option for management. Other options include Aquashade, a vegetable-based dye or pigment, used in ponds to reduce light passing through the water to limit algal growth. There are also some peroxide compounds like GreenClean that can manage algae. Algae growth can take hold because of an excess of nutrients in the water.
Penn State Extension's Pond Management website has a lot more information about pond management, including strategies to avoid excess nutrients in the water.
Sulfuric acid, phosphoric acid, nitric acid, citric acid, and acetic acid can be used to lower irrigation water pH. Deciding which one to use is often dependent on availability, price, and growing methods. Many farmers use sulfuric acid because it is readily available and relatively inexpensive. However, nitric and phosphoric acid have the advantage of adding nitrogen and phosphorus to the water, respectively. Sulfuric, phosphoric, and nitric acids are industrial by-products and not allowed in certified organic production. Organic farmers have the options of citric acid and/or acetic acid (check with your certifier before using any product on an organic farm to ensure the product does not compromise certification).
Alkalinity should not be confused with hardness. Hardness is a combined measure of carbonates (alkalinity) and non-carbonates (iron, chlorides, sulfates, etc.). While carbonate hardness can be treated with acid, non-carbonate hardness cannot. From a plant perspective, hardness is not an issue. However, it is a problem in boilers and pipes and can reduce the effectiveness of some pesticides (i.e., insecticidal soaps).
Salinity is another measure of irrigation water quality to consider. Total dissolved solids, given in milligrams per liter (mg/l), measures the number of particles dissolved in the water, including inorganic nutrients. Electrical conductivity, given in millimhos per centimeter (mmhos/cm), measures the soluble salts level.
One early symptom of high values (for total dissolved solids above 640 for plugs/seedlings or above 960 for others and for electrical conductivity above 1.0 for plugs or above 1.5 for others) is that the margins of leaf tissue can die.
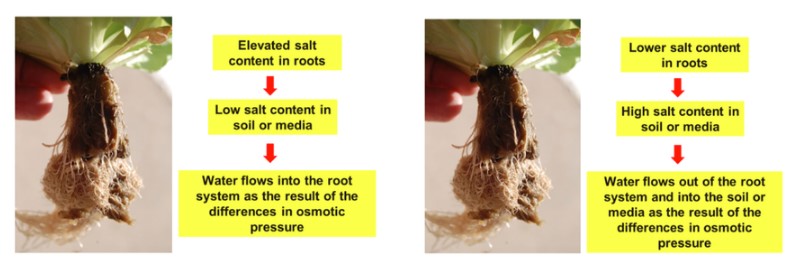
Chemical induced drought can also occur with very high values. Water flows from soil or media as a result of differences in osmotic potential: water flows from areas of low salt concentration to levels of high salt concentration. Normally the salt content of the roots is higher than the soil or media and therefore water flows from the soil or media to the roots. When salt levels are excessive in the soil or media due to over application of fertilizer or using water with high total dissolved solids or electrical conductivity, water can flow from the roots to the soil or media. In this case, plants can wilt and die.
Water treatment options generally include reverse osmosis, de-ionization, distillation, dilution, and acidification. Acidification is used to lower pH as described above. Reverse osmosis, de-ionization, and distillation can be used to correct high sodium, chloride, total dissolved solids, and electrical conductivity levels as well as sodium adsorption ratios. However, they are costly fixes not commonly used by vegetable farmers. When values are not too high, dilution with rain water can be an effective solution for these same issues.