We begin the series today with a discussion of the differences between biodiesel and renewable diesel fuels. Currently, biomass-based diesel fuels are produced from organic lipid feedstocks that contain free fatty acids, such as vegetable oils, animal fats, or recycled grease (AFDC, 2022a,b). While biodiesel and renewable diesel are both categorized as biomass-based diesel fuels and can be produced using the same feedstock, they are distinctly different types of fuels that differ in several important characteristics.
Biodiesel
Biodiesel, often referred to as FAME biodiesel or simply FAME, is produced by a process known as transesterification (FAME stands for Fatty Acid Methyl Ester). Transesterification converts organic fats and oils into fatty acid alkyl esters by reacting them with alcohols and catalysts (AFDC, 2022a). Methanol typically serves as the reactant and creates fatty acid methyl esters. During the transesterification process, 100 pounds of vegetable oil or fat is typically reacted with 10 pounds of methanol to produce about 100 pounds of biodiesel and 10 pounds of glycerol. Equivalently, about 7.5 pounds of feedstock are required to produce one gallon of biodiesel and 0.9 pounds of glycerin (farmdoc daily, February 15, 2022). Several uses exist for both crude and refined glycerol, typically called glycerin, with a glycerol content of around 80 percent. Glycerol can be used in animal feeds or a variety of chemical processes.
A schematic of the FAME biodiesel production process is provided in Figure 1. The schematic represents what is known as a “continuous” production process. Batch processes are also used in older and smaller biodiesel plants. The steps in the production process are similar for both types of processes, except the reactor phase is much longer in the batch process.
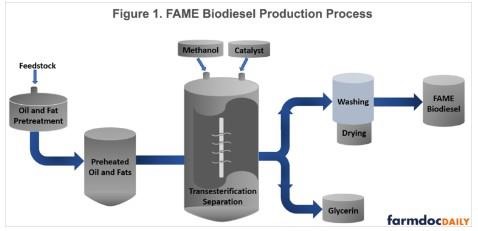
As shown in Figure 1, the feedstock is first pre-treated to remove impurities. The treated feedstock is then pre-heated to around 130 degrees Fahrenheit. While the feedstock is preheating, its quality is tested for free fatty acid content. Keeping the temperature steady, feedstock is then loaded into the reactor along with methanol and base catalysts and, rarely in the U.S., enzymes. The mixture is then stirred until the chemical reaction is complete, often in as little as an hour. After the reaction is complete, the reaction mixture is allowed to settle for several hours. During this time, glycerin and biodiesel are separated, with glycerin settling to the bottom of the reactor and biodiesel to the top due to the difference in their density. When the settling time passes, the glycerol is separated from the methyl esters by draining from the bottom of the reactor. The glycerin is purified by removing the excess methanol, and then stored in a tank. The methyl esters are transferred into a different tank where they are washed with water, many times until the water runs clear. The washing removes any left-over catalyst, soap, salt, methanol, and remaining glycerin from methyl esters. After washing, the methyl esters undergo an extensive drying process until the remaining moisture is removed completely. Finally, the fatty acid methyl ester (FAME) biodiesel is stored in tanks ready for consumption.
Pure FAME biodiesel created from this process is known in the industry as B100. It is important to emphasize that FAME is not a hydrocarbon fuel like petroleum diesel, and therefore, differs from petroleum diesel in several key aspects (Brown, 2020). First, FAME biodiesel contains oxygen, which causes FAME to have approximately seven percent less energy by volume. Second, the higher oxygen content of FAME can limit the length of time it can be stored due to oxidation that causes corrosion. Third, FAME’s chemical composition can make it more susceptible to microbial fouling when poor storage tank management is present, which may result in the corrosion of storage tanks and clogging of fuel lines. Fourth, FAME biodiesel has a relatively high temperature where it will begin to freeze and form visible crystals. This is known in technical terms as the cloud point of the fuel. For example, the cloud point for FAME biodiesel made from soybean oil is approximately 34 degrees Fahrenheit compared to around 16 degrees Fahrenheit for petroleum diesel. If the freezing process continues, the fuel will eventually solidify completely, a condition known as “gelling.” Once gelling occurs, the solidified fuel will not flow through fuel lines. For these reasons, FAME biodiesel is blended with petroleum diesel for final consumption. Common blends include B5 (up to 5 percent biodiesel) and B20 (6 to 20 percent biodiesel).
In addition to some of the challenges facing the use of biodiesel, it provides other positive aspects for users. For example, biodiesel has desirable lubricity characteristics due to its chemical composition. This feature helps prevent premature engine wear.
Renewable Diesel
Renewable diesel, sometimes referred to as hydrotreated vegetable oil (HVO) or green diesel, is produced using several production processes. In the U.S, the most common process is known as hydroprocessing or hydrotreating (AFDC, 2022b). The hydrotreating process parallels the process used to “crack” crude oil into gasoline, diesel, and other petroleum products in a crude oil refinery. Consequently, renewable diesel production facilities are increasingly converted parts of crude oil refineries or complete conversions of refineries. Some are entirely new refinery facilities. The potential to switch between crude oil and organic fats and oils refining exists for all technologies if economic conditions dictate. Because crude oil refining technology is used to produce renewable diesel, the capital costs are substantially higher for renewable diesel production compared to FAME production.
Figure 2 presents a schematic of renewable diesel production using hydrotreating. Like the FAME production process, impurities must be removed before beginning the refining process. The next step is the entrance of treated renewable feedstock and hydrogen into the hydrotreating reactor, where they flow over a bed of solid particulate catalyst. At this stage, the catalyst triggers reactions of hydrogen with the feedstock under high temperature and pressure, forming mostly water and liquid hydrocarbon molecules that are suitable for use as fuel with additional processing. After the hydrotreatment, the reactor product mixture moves to a separation unit where steam and other light product gasses are removed as vapor and the hydrogenated liquid hydrocarbon leaves the process, mostly consisting of long-chain alkanes. Then, the liquid stream passes through a series of distillation columns, separating the products by their boiling points. In addition to producing renewable diesel, this also yields co-products such as fuel gas, LPG and naphtha. The feedstock requirements for renewable diesel production are greater than for biodiesel and vary depending on the final proportions of the co-products desired from the production process. The reason feedstock requirements are higher is that a greater amount of material is lost during the renewable diesel production process. While there is a great deal of variation in the renewable diesel production industry, a reasonable benchmark is that approximately 8 pounds of feedstock is used to produce 1 gallon of renewable diesel and minor amounts of naphtha and propane (Xu, et. al., 2022).
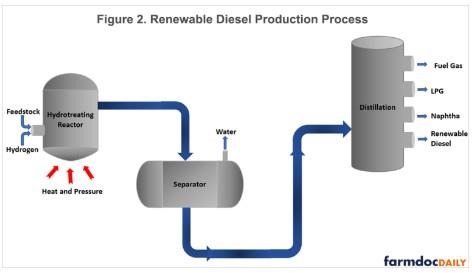
Renewable diesel is fundamentally different from FAME biodiesel, in that it only contains hydrogen and carbon, making it a hydrocarbon fuel just like petroleum diesel. While not identical to petroleum diesel, renewable diesel is so close that it is considered a “drop-in” replacement for petroleum diesel. In other words, renewable diesel does not need to be blended with petroleum diesel for it to be used as a “spec” fuel in modern diesel engines. The drop-in nature of renewable diesel is a significant advantage over FAME biodiesel. Renewable diesel does have a somewhat lower energy content compared to petroleum diesel (four percent less by volume), but this may be offset by other desirable properties of renewable diesel (Brown, 2020).
Finally, note that blended products do exist, such as 5 percent renewable diesel with 95 percent petroleum diesel (R5) and 20 percent renewable diesel with 80 percent petroleum (R20) (EIA, 2020). If renewable diesel is blended, the two fuels are so similar that carbon dating is required to distinguish between the fossil carbon in petroleum diesel and organic carbon in renewable diesel (Brown, 2020). It should also be noted that it is not unusual for a small amount of biodiesel to be blended with renewable diesel for lubricity purposes.
Implications
The boom in renewable diesel production has raised a whole host of questions about the potential impacts on U.S. agriculture. In order to answer these questions, it is crucial to first have a clear understanding of how renewable diesel differs from FAME biodiesel, historically the more important of the two renewable fuels. While the same organic fats and oil feedstocks are used to make both fuels, the production processes are radically different. Biodiesel uses a relatively simple chemical reaction production process. For this reason, biodiesel is normally blended with petroleum diesel to be used in modern diesel engines. In contrast, renewable diesel is fully refined and cracked using petroleum refining technology. This results in a “drop-in” hydrocarbon fuel that meets the same technical specifications as petroleum diesel, and as such, can be used as a complete replacement for petroleum diesel.
The next article in this series will examine the basic economic framework for biodiesel and renewable diesel markets and the policies that provide support for the sector.
Source : illinois.edu